The Role of HPLC in the Detection and Characterisation of Viruses
29 Jun 2020
HPLC is an established technique for the quantification and characterisation of viral particles in vaccine development, and identifying and characterising the proteins corresponding to different viral serotypes when looking at the epidemiology of disease.1-3 How do these techniques work, and how can we ensure that they are as accurate as possible when it comes to their most important solvent: ultrapure water?
A virus typically consists of a nucleic acid molecule in a protein coat and can only multiply within the living cells of a host. Viruses are often mistakenly referred to as “alive”, although the correct term is “active”, since they are parasites that cannot survive in their “active” form outside of their host organism.4 They are much smaller than bacteria. E.g. With a diameter of 220 nm, the measles virus is about 8 times smaller than E.coli, and the family of coronaviruses is even smaller, with an average diameter of around 120 nm.5
The viral capsid, or coat, has characteristic proteins, which must elicit an immune response (antibody response) in the host organism in order to be eradicated, lest they go on to produce their associated disease, spreading via droplet infection in the host population.6 The word “mutation” is whispered in hushed tones, referring to the tiny changes in these coat proteins which enable a given virus to escape detection by the immune system and continue its rampage through the body of the host e.g. the ‘flu’ virus mutates so often that a new vaccine has to be developed every year, with researchers taking their lead from epidemiologists, regarding which strains will prevail in a particular region in a given year (Fig. 1).7
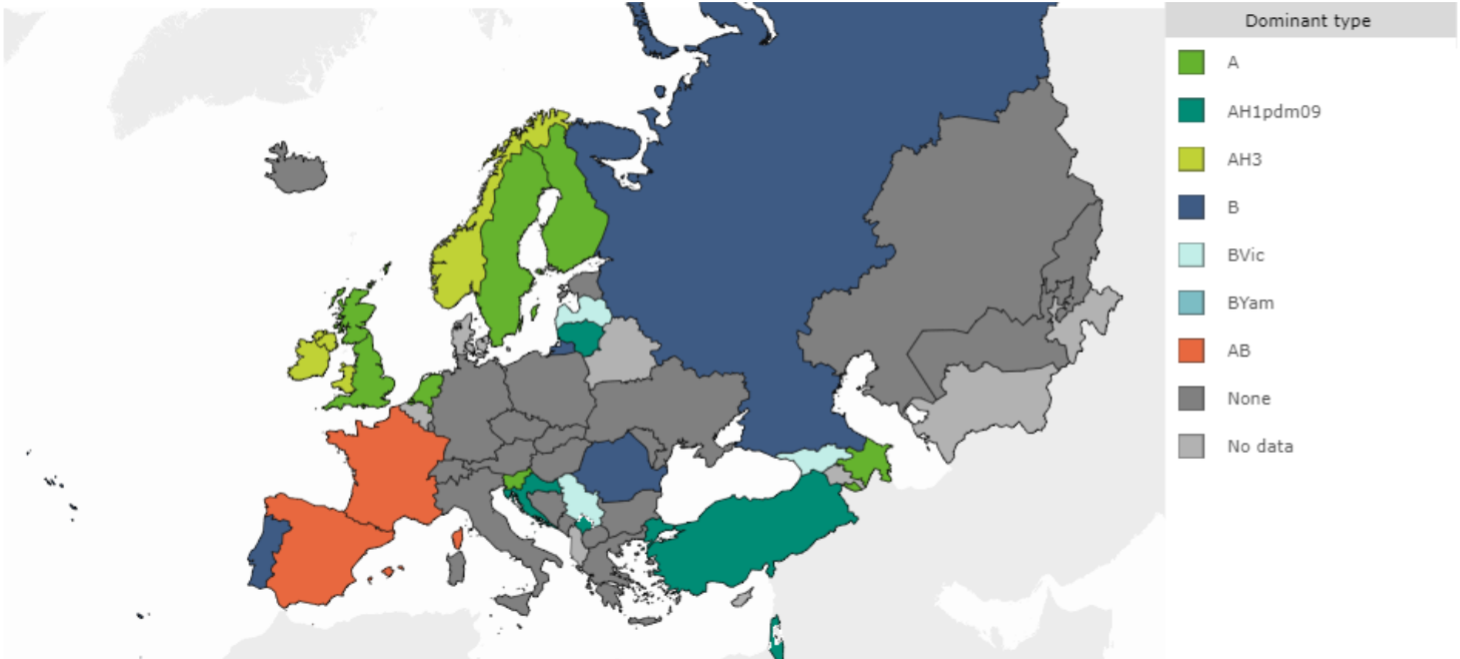
Figure 1. This map of the prevalent WHO strains of influenza virus in European Region, week 49, 2019, demonstrates how difficult it can be to predict and recommend a common vaccine for a given season.
One reason why HPLC and LC/MS are so useful for the characterisation of viruses is that they obviate the need for raising specific antibodies and the development of ELISA tests: the viral proteins, or portions of them, known as peptides, are simply separated on an HPLC column, and analysed directly or after mass spectrometry.8,9
The nature and degree of separation of the component parts of the viral protein coat will give an indication of the structure of the virus, and is so accurate that separate serotypes can be detected, as seen in the case of influenza virus.1
HPLC can thus be considered as a key technique in the investigation of disease outbreaks, allowing the characterisation of the species and sub-species of a virus that are found in individual patients, and for detailed epidemiological maps to be constructed. Such maps are constructed on a continuous basis for following diseases such as influenza around the planet.10
It is not only viruses which can be characterised in this way. HPLC and LC/MS can also be used to determine the specific molecular fingerprints for different subspecies of bacteria, particularly important when it comes to tracing the sources of hospital infections and for tracking antibiotic resistance.11,12
When it comes to detecting and quantifying the amount of protein or peptide present in a given patient sample, which can then be extrapolated to quantifying the viral load and characterising the specific viral subtype, very often HPLC and LC/MS are working at the limits of detection.1,13 It is therefore imperative that the solvents used in the preparation of the samples and for the chromatographic runs themselves are as pure as possible. This includes the water used to prepare samples and buffers throughout the entire workflow. The use of ultrapure water will avoid such issues as ghost peaks interfering with an already tiny spike in a chromatogram, which could prompt a false positive result.14 The importance of pure water when working at the limits of the HPLC and LC/MS assay detection is further explained in our white paper, where we look in more depth at the development of optimised assays for such diverse applications as therapeutic drug monitoring and forensic toxicology.
Vaccination: How HPLC is Used in the Manufacture of Viral Vectors
We saw above how HPLC and LC/MS assays have been effectively developed and optimised for the quantification and characterisation of viruses and bacteria when investigating the epidemiology of specific diseases. This begs the question: if it is possible to use HPLC to detect viruses in such low amounts, and even characterise their individual sub-species, might HPLC also be useful when we are developing virus-based vaccines?
No story of viral-based vaccine development would be complete without paying tribute to Edward Jenner, considered the founder of vaccinology when in 1796, he inoculated a 13 year-old-boy with vaccinia virus (cowpox), and demonstrated immunity to smallpox. In 1798, the first smallpox vaccine was developed. The next routinely recommended vaccines were not developed until early in the 20th century.16 These included vaccines that protect against whooping cough (pertussis, 1914), diphtheria (1926), and tetanus (1938), all three of which were combined in 1948 and given as the DTP vaccine.15 The polio vaccine was licensed in 1955, in 1963 the measles vaccine was developed, and by the late 1960s, vaccines were also available to protect against mumps (1967) and rubella (1969).15 These three vaccines were combined into the MMR vaccine in 1971.15
In the final decades of the 20th century, as molecular biology developed and evolved, first into a science and then into a routine tool, scientists started to study how viral particles hijack the genetic machinery of the human cell, and to look at the possibility of using them to develop further vaccines. What if they could attenuate specific viral particles and use them as a means to introduce recombinant nucleic acids into the human body? What if they could, in so doing, get the body to make the proteins corresponding to those nucleic acids, and thus elicit an immune response, to immunise against disease? And the idea of viral vectors was born.
Since they can effectively induce both humoral and cell-mediated immune responses, viral vectors are a great alternative to the traditional platforms to deliver vaccine antigens corresponding to specific diseases, as well as to specifically target and kill cancer cells. The benefits resulting from successful application of viral vectors to prevent and treat human diseases are potentially huge. Indeed, this is the promising approach used in labs around the world in the race to find vaccines against any number of diseases, including the most recent coronavirus, SARS-CoV-2, or COVID-19.16
Adenovirus is a common viral vector that is considered a workhorse when it comes to developing these ambitious-sounding recombinant vaccines.17 Adenoviruses (Ads) have been extensively studied for their potential use in gene therapy applications. Owing to years of research, which has established a foundation for the use of this linear double-stranded DNA virus as a vector for vaccine delivery, Ads have become one of the most exploited vectors for vaccine development.
Major advantages of using Ads as a vaccine platform include their ability to infect a broad range of hosts, and to induce high levels of transgene expression, without the potential of viral genes being integrated into the host genome. Importantly, due to their ability to grow in high titres in cell culture, Ads can be manufactured safely and inexpensively.17
Let us take an example of such research in the UK. As soon as the genome sequence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became available in mid-January, Sarah Gilbert’s Oxford team set to work to design a vaccine, using recombinant DNA techniques to create a SARS-CoV-2 antigen, and embedding it within a primate adenovirus vector. The vaccine contains the genetic sequence of the “spike” protein found on the outside of the coronavirus. After vaccination, the surface spike protein of the coronavirus is produced, which primes the immune system to attack the coronavirus if it later infects the body. A chimpanzee adenovirus vaccine vector (ChAdOx1) was chosen as the most suitable vaccine technology for a SARS-CoV-2 vaccine. This is because it can generate a strong immune response from one dose and it is not a replicating virus, so it cannot cause an ongoing infection in the vaccinated individual. This also makes it safer to give to children, the elderly and anyone with a pre-existing condition such as diabetes. Trials of this vaccine begin in May 2020, with the possibility of a vaccine becoming available later in the year.
HPLC and LC/MS are not only used in the characterisation of the recombinant proteins produced by viral vectors, they are also essential techniques throughout the vaccine manufacturing workflow, where they are used for quantification and quality control, alongside gel-based and PCR-based methods. The steps involved in purification of adenovirus are (1) cell lysis and genomic DNA breakdown, (2) clarification (3) concentration with ultrafiltration/ diafiltration, (4) anion-exchange (AEX) purification, (5) gel filtration, and (6) microfiltration.18 HPLC can be used to QC any or all of these stages, and is particularly important to ensure removal of all contaminants at the end of the process, where it will be working at the limits of detection.
Conclusion
The importance of high quality HPLC and LC/MS assays in our fight against disease cannot be understated, whether characterising a disease in the first place or monitoring the production of vaccines directed towards their potential cure. We are so often using HPLC at the lower extremes of detection, whether of viral particles/proteins in disease, or of contaminants in the vaccines, that we must ensure ultrapure water is used at all stages of research and manufacture. To learn more about this, and the application of HPLC in pharma and LC/MS, download our white paper/s.
Download our White Paper HPLC in pharma / Reducing risk in LC/MS
References
1. Transfiguracion, J., Manceur, A. P., Petiot, E., Thompson, C. M., & Kamen, A. A. (2015). Particle quantification of influenza viruses by high performance liquid chromatography. Vaccine, 33(1), 78–84. https://doi.org/10.1016/j.vaccine.2014.11.027
2. Jin X., Liu L., Nass S., O'Riordan C., Pastor E. and Zhang X.K. (2017) Hum Gene Ther Methods. 28(5):255-267.
3. Zimmermann, A., Mertens, T., Schnlz, A., Kruppenbacher, J.P., Nelsen-Salz, B. and Eggers, H.J. (1993) Journal of General Virology, 74, 2759-2763.
4. https://www.medicinenet.com/script/main/art.asp?articlekey=5997 accessed 20 May 2020.
5. https://www.britannica.com/science/virus/Size-and-shape accessed 20 May 2020.
6. Gahéry-Ségard, H., Farace, F., Godfrin, D., Gaston, J., Lengagne, R., Tursz, T., Boulanger, P., & Guillet, J. G. (1998). Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. Journal of virology, 72(3), 2388–2397.
7. https://www.cdc.gov/flu/prevent/vaccine-selection.htm accessed 20 May 2020.
8. Xie, H., Doneanu, C., Chen, W., Rininger, J., & Mazzeo, J. R. (2011). Characterization of a recombinant influenza vaccine candidate using complementary LC-MS methods. Current pharmaceutical biotechnology, 12(10), 1568–1579. https://doi.org/10.2174/138920111798357447
9. Li, Z., Sun, W., Wu, D., Gao, X., Sun, N., & Liu, N. (2014). Mass spectrometry analysis coupled with de novo sequencing reveals amino acid substitutions in nucleocapsid protein from influenza A virus. International journal of molecular sciences, 15(2), 2465–2474. https://doi.org/10.3390/ijms15022465
10. https://www.ecdc.europa.eu/sites/default/files/documents/influenza-situation-assessment-18-December-2019.pdf Accessed 20 May 2020.
11. Franco-Duarte, R., Černáková, L., Kadam, S., Kaushik, K. S., Salehi, B., Bevilacqua, A., Corbo, M. R., Antolak, H., Dybka-Stępień, K., Leszczewicz, M., Relison Tintino, S., Alexandrino de Souza, V. C., Sharifi-Rad, J., Coutinho, H., Martins, N., & Rodrigues, C. F. (2019). Advances in Chemical and Biological Methods to Identify Microorganisms-From Past to Present. Microorganisms, 7(5), 130. https://doi.org/10.3390/microorganisms7050130
12. Vestergaard, M., Frees, D., & Ingmer, H. (2019). Antibiotic Resistance and the MRSA Problem. Microbiology spectrum, 7(2), 10.1128/microbiolspec.GPP3-0057-2018. https://doi.org/10.1128/microbiolspec.GPP3-0057-2018
13. Sharma, V.K., Sharma, I. & Glick, J. (2018) The expanding role of mass spectrometry in the field of vaccine development Wiley Mass Spectrometry Reviews. DOI: 10.1002/mas.21571
14. https://www.elgalabwater.com/hplc-ultrapure-water Accessed 20 May 2020
15. Vaccine history review ref https://www.chop.edu/centers-programs/vaccine-education-center/vaccine-history/developments-by-year
16. Choi, Y., & Chang, J. (2013). Viral vectors for vaccine applications. Clinical and experimental vaccine research, 2(2), 97–105. https://doi.org/10.7774/cevr.2013.2.2.97
17. Sarah Gilbert: carving a path towards a COVID-19 vaccine Crossref DOI link: https://doi.org/10.1016/S0140-6736(20)30796-0 Perspectives. Sarah Gilbert: carving a path towards a COVID-19 vaccine www.thelancet.com Vol 395 1247 April 18, 2020
18. Vellinga,J. et al., (2014) Human Gene Therapy 25:318–327 (April 2014) Mary Ann Liebert, Inc. DOI: 10.1089/hum.2014.007
